Ale 25 the First Law of Thermodynamics Answers
Home Chemistry Thermodynamics First Law of Thermodynamics Give the comparison of work of expansion of an ideal Gas and a van der Waals Gas. The first law of thermodynamics states that.
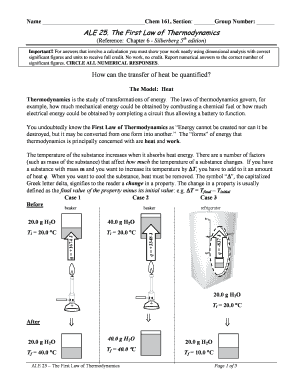
Ale 25 The First Law Of Thermodynamics Fill Online Printable Fillable Blank Pdffiller
2000 J of heat is added to a system and 2500 J of work is done on the system.

. Hence for the expansion of a gas V2 V1 which shows. Internal energy increases by 500 Joule. W work interaction of the system with its surroundings.
SECOND LAW OF. No work no credit. Energy is constantly being created by entropy events.
Since energy is conserved in all actions the change in a systems energy can be equated to the net transport of energy into the system. Solution for define first law of thermodynamics hesss law and bond energy. 6 Questions Show answers.
You undoubtedly know the First Law of Thermodynamics as Energy cannot be created nor can it be destroyed but it may be converted from one form into another The forms of energy that thermodynamics is principally concerned with are heat and work. Nowadays the law of conservation of energy or First Law of Thermodynamics is often derived from Noethers Theorem but that is some advanced mathYou can convert one type of energy to another. For answers that involve a calculation you must show your work neatly using dimensional analysis with correct significant figures and units to receive full credit.
If the system as a whole is at rest so that the bulk mechanical energy due to translational or rotational motion is. The First Law of Thermodynamics Reference. There are a number of factors.
Heat gained heat lost E. The First Law of Thermodynamics states. In this chapter we discover that the first law of thermodynamics is simply the conservation of energy principle.
The temperature of the substance increases when it absorbs heat energy. However energy can certainly be transferred from one part of the universe to another. What is the equation for the first law of thermodynamics.
Through countless experiments we discover that the amount of energy at the beginning of any experiment is equal to the amount of energy at the end of the experiment. Suppose that a closed system of unit mass takes in a certain quantity of thermal energy q which it can receive by ther-. Now the conservation of energy principle or the first law of thermodynamics for closed systems is written as QW U KE PEnet net If the system does not move with a velocity and has no change in elevation the conservation of energy equation reduces to QW Unet net.
That is energy can neither be created nor destroyed. Wideal -nRT ln V2 V1 And for a a van der Waals Gas work done is given as. The first law of thermodynamics is the familiar conservation of energy principle.
FIRST LAW OF THERMODYNAMICS the total heat energy applied to the system is equal to the sum of the change in internal energy of the system and the work done by the system. By applying this law deduce the relation between the two specific heats of gas. The first law of thermodynamics is that energy can not be created or destroyed but it can be changed from one form to another.
To play this quiz please finish editing it. An unknown piece of metal weighing 950 g is heated to 980 o C. The energy of motion or that can be used to do work is known as.
ΔU q W. Cells USE energy but do not create it. Energy can be changed from one form to another but.
3000 J of heat is added to a system and 2500 J of work is done by the system. The _______ of energy would break the first law of thermodynamics. Chapter 6 - Silberberg sedition Important.
Where ΔU change in internal energy of the system. The First Law of Thermodynamics Work and heat are two ways of transfering energy between a system and the environment causing the systems energy to change. The First Law of Thermodynamics A mass of gas possesses internal energy due to the kinetic and potential energy of its molecules or atoms.
We know that for an ideal gas work done w is given as. The equation for the first law of thermodynamics is given as. ALE 25 The First Law of Thermodynamics Page 5 of 5 b Assuming the heat capacity of the calorimeter is negligible calculate the maximum final temperature of the system.
State the first law of thermodynamics. Energy cannot be created or destroyed. The first law of thermodynamics problems and solutions.
Report numerical answers to the correct number of significant figures. Q algebraic sum of heat transfer between system and surroundings. It is dropped into 2500 g of water at 230 o C.
The 1st Law of Thermodynamics tells us that energy is neither created nor destroyed thus the energy of the universe is a constant. Changes in internal energy are manifested as changes in the tempera-ture of the system. What is the change in internal energy of the system.
It states that when a certain amount of heat energy is supplied to a system some part of it is used to perform external work and rest of heat is used to increase the internal energy of the system. First Law of Thermodynamics Equation.

Ale 25 The First Law Of Thermodynamics Reference Chegg Com


No comments for "Ale 25 the First Law of Thermodynamics Answers"
Post a Comment